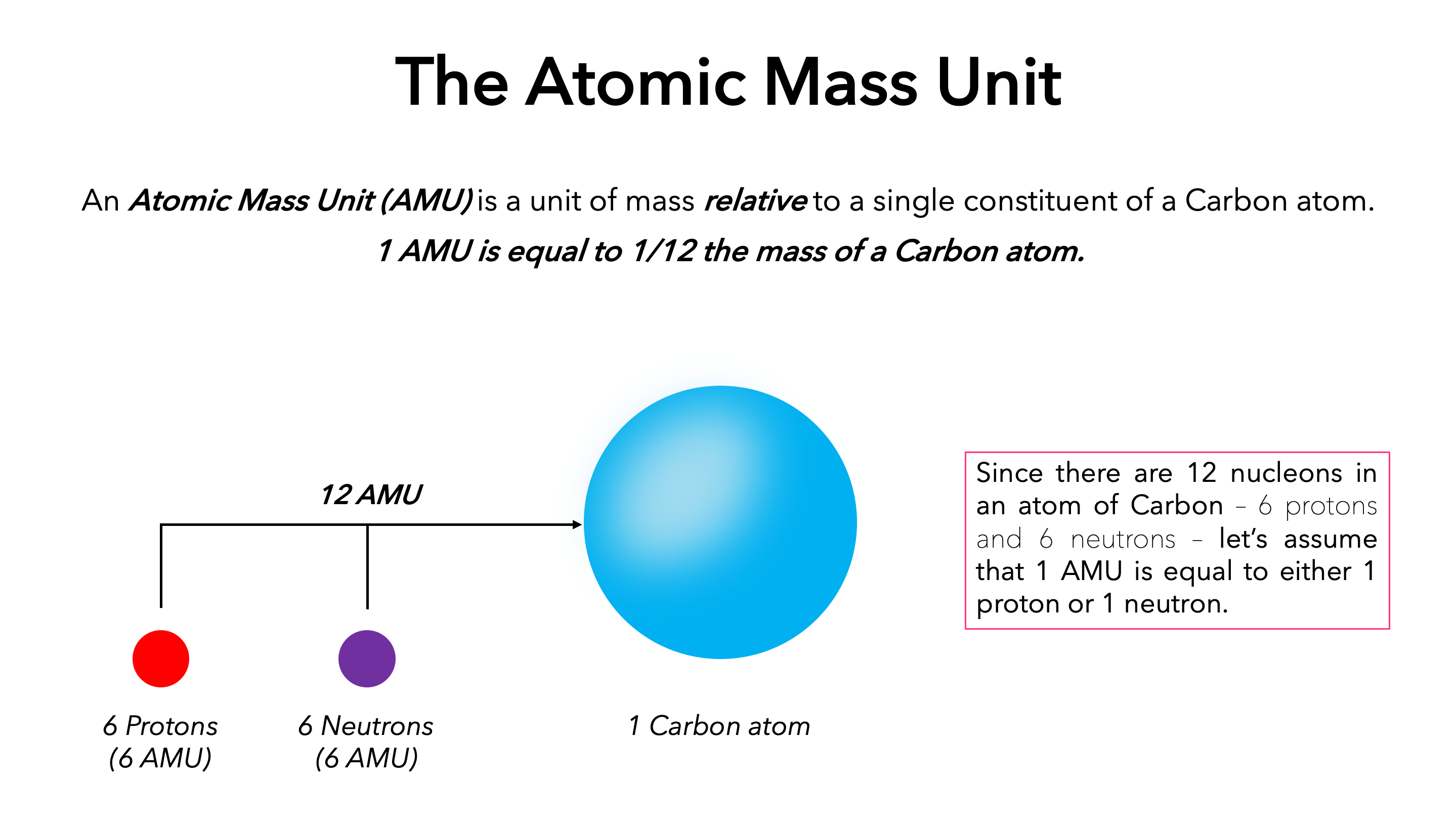
Assertion : One atomic mass unit (amu ) is mass of an atom equal to exactly one twelfth the mass of a carbon - 12 atom . Reason : Carbon - 12 isotope was selected as standard. - Sahay Sir

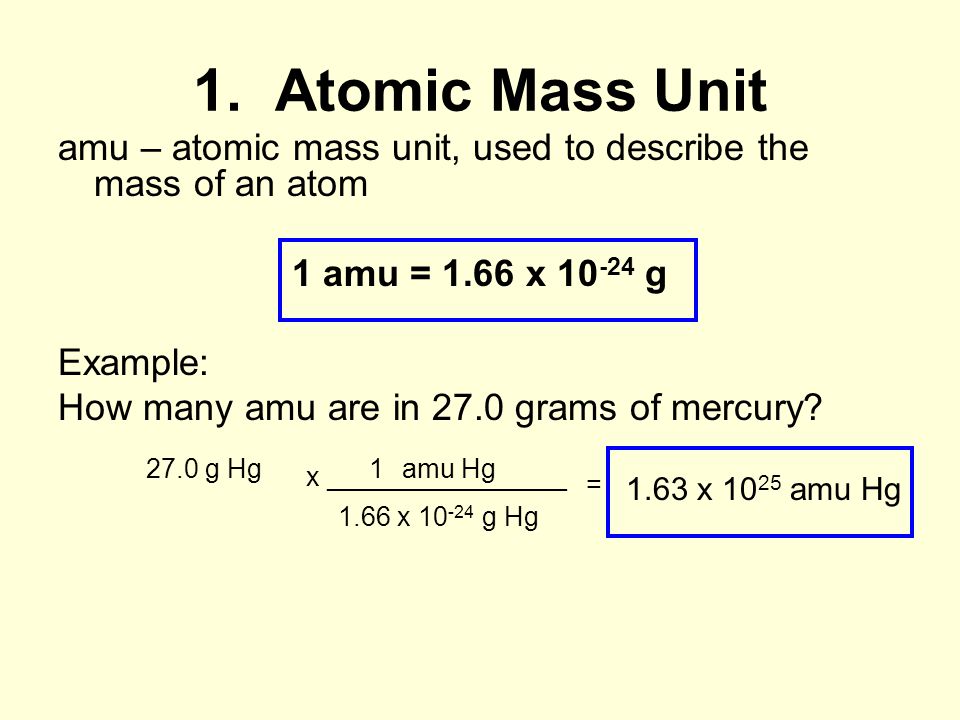
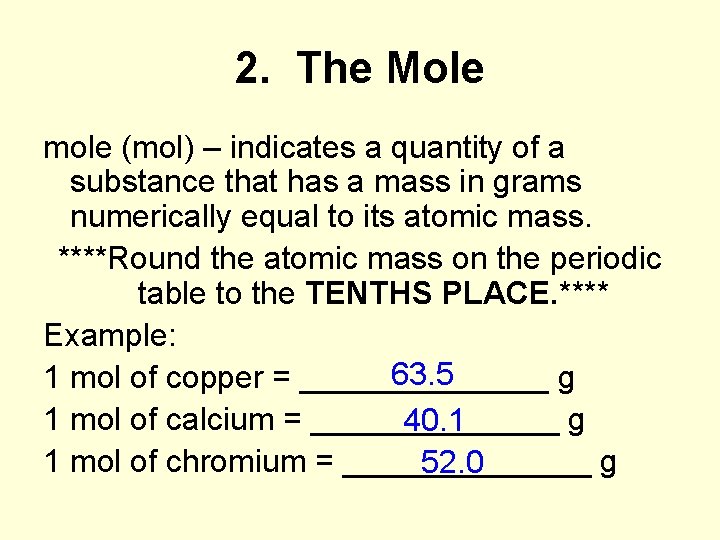
Moles Notes. 1. Atomic Mass Unit amu – atomic mass unit, used to describe the mass of an atom Conversion factor: 1 amu = 1.66 x g Equivalence statement: - ppt download

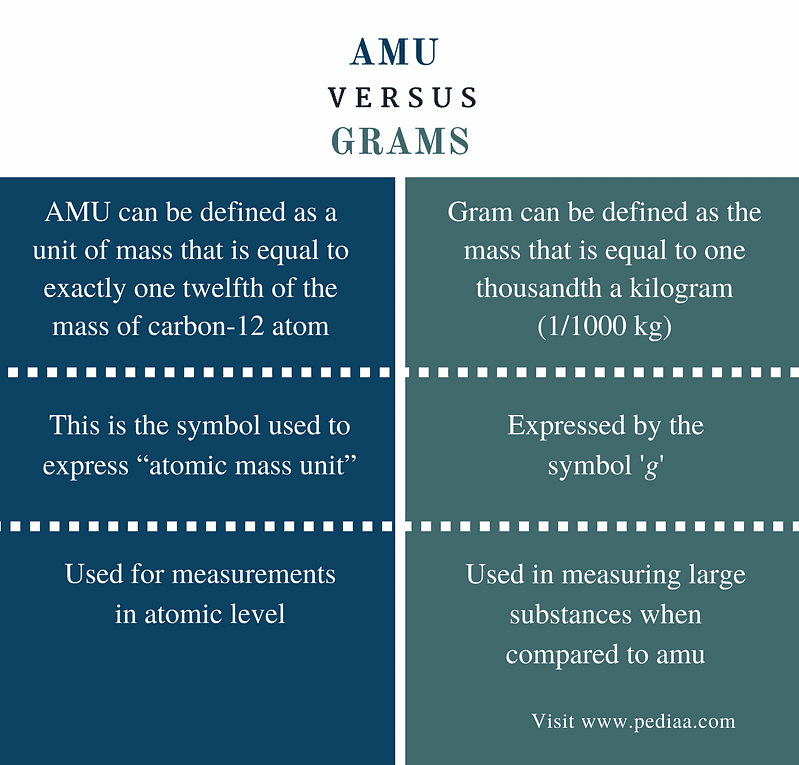
What is the distinction between 1 dalton and 1 amu in the new SI definitions, voted in November 16? - Quora





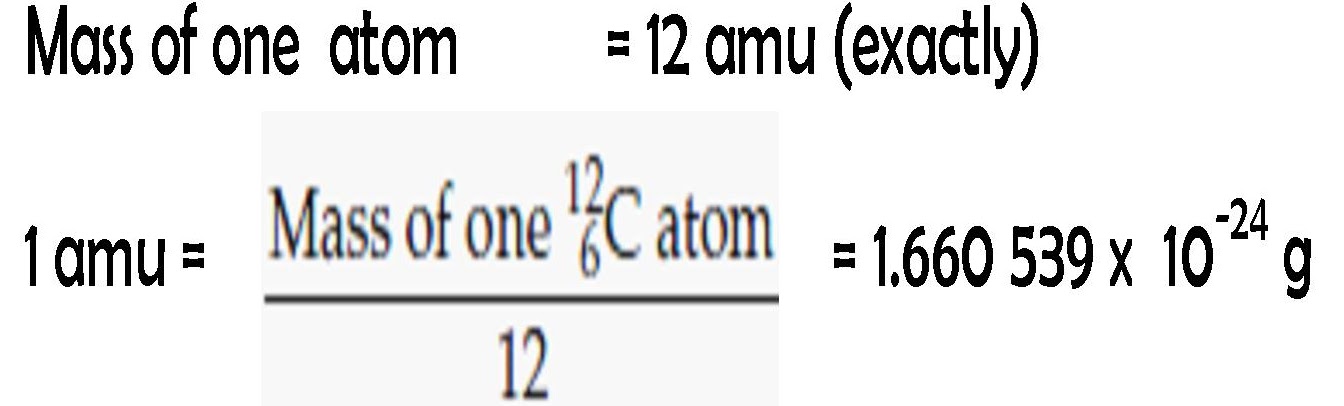







/hands-cupping-a-glowing-atom-in-the-studio-164210758-5b259c6b04d1cf0036d0b90f.jpg)