Description of prototype modes-of-action related to repeated dose toxicity - Publications Office of the EU

Figure 1 from In Silico Models for Repeated-Dose Toxicity (RDT): Prediction of the No Observed Adverse Effect Level (NOAEL) and Lowest Observed Adverse Effect Level (LOAEL) for Drugs. | Semantic Scholar

Table 1 from Screening of repeated dose toxicity data present in SCC(NF)P/SCCS safety evaluations of cosmetic ingredients | Semantic Scholar
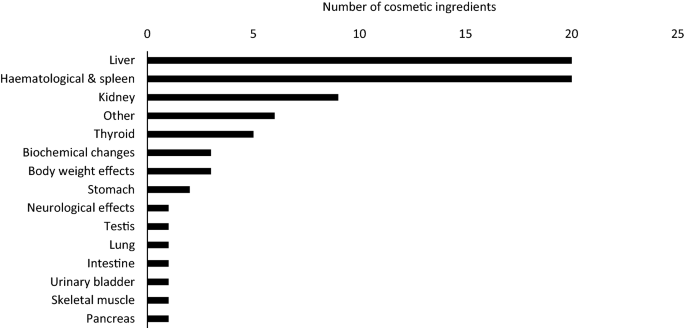
Screening of repeated dose toxicity data in safety evaluation reports of cosmetic ingredients issued by the Scientific Committee on Consumer Safety between 2009 and 2019 | SpringerLink
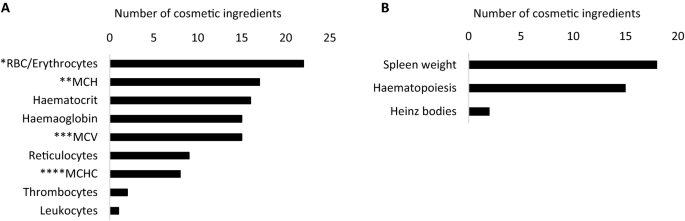
Screening of repeated dose toxicity data in safety evaluation reports of cosmetic ingredients issued by the Scientific Committee on Consumer Safety between 2009 and 2019 | SpringerLink

THE SINGLE–AND REPEATED–DOSE TOXICITY OF DIMETHYL SULFOXIDE - Smith - 1967 - Annals of the New York Academy of Sciences - Wiley Online Library

Figure 1 | Modes-of-Action Related to Repeated Dose Toxicity: Tissue-Specific Biological Roles of PPARγ Ligand-Dependent Dysregulation in Nonalcoholic Fatty Liver Disease

Read-across of 90-day rodent repeated-dose toxicity: A case study for selected simple aryl alcohol alkyl carboxylic acid esters - ScienceDirect
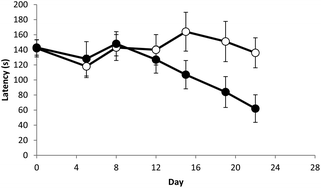
Functional assessments in repeat-dose toxicity studies: the art of the possible - Toxicology Research (RSC Publishing)

Acute and repeated doses (28 days) oral toxicity study of Vicenin-1, a flavonoid glycoside isolated from fenugreek seeds in laboratory mice - ScienceDirect










