Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials. - Abstract - Europe PMC

Acute Kidney Injury and Electrolyte Abnormalities After Chimeric Antigen Receptor T-Cell (CAR-T) Therapy for Diffuse Large B-Cell Lymphoma - American Journal of Kidney Diseases

Acute Kidney Injury and Electrolyte Abnormalities After Chimeric Antigen Receptor T-Cell (CAR-T) Therapy for Diffuse Large B-Cell Lymphoma - American Journal of Kidney Diseases

Emerging Treatment Options for Acute Lymphoblastic Leukemia: Focus on CAR T-Cell Therapy in: Journal of the National Comprehensive Cancer Network Volume 16 Issue 5S (2018)

Acute Kidney Injury and Electrolyte Abnormalities After Chimeric Antigen Receptor T-Cell (CAR-T) Therapy for Diffuse Large B-Cell Lymphoma - American Journal of Kidney Diseases

Acute Kidney Injury and Electrolyte Abnormalities After Chimeric Antigen Receptor T-Cell (CAR-T) Therapy for Diffuse Large B-Cell Lymphoma - American Journal of Kidney Diseases

ASCO: Gilead's Kite soars over Novartis' CAR-T turf with Tecartus win in type of leukemia | Fierce Pharma

ثلاثون زاوية عار novartis car t advisory committee briefing documents - healthiercitiescommunities.com

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials. - Abstract - Europe PMC
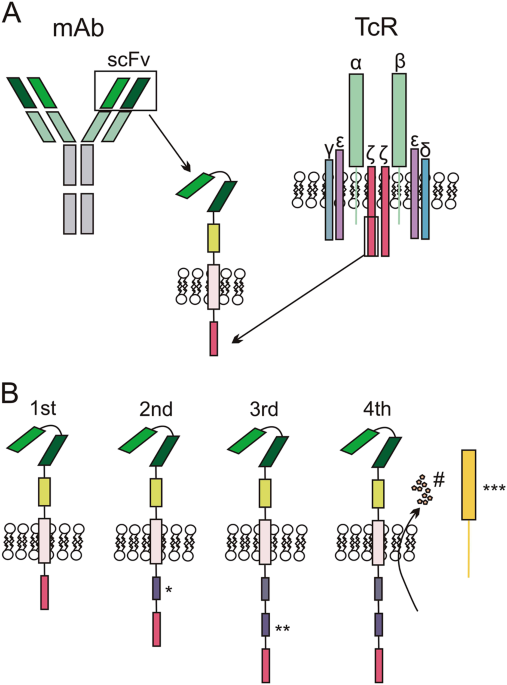
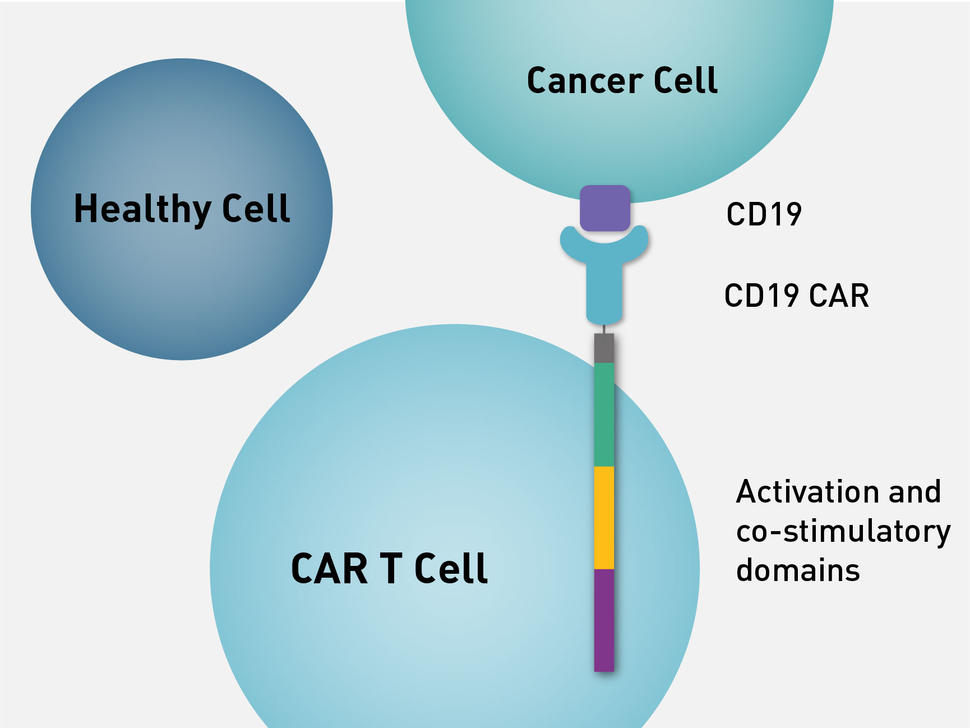
The biological basis and clinical symptoms of CAR-T therapy-associated toxicites | Cell Death & Disease
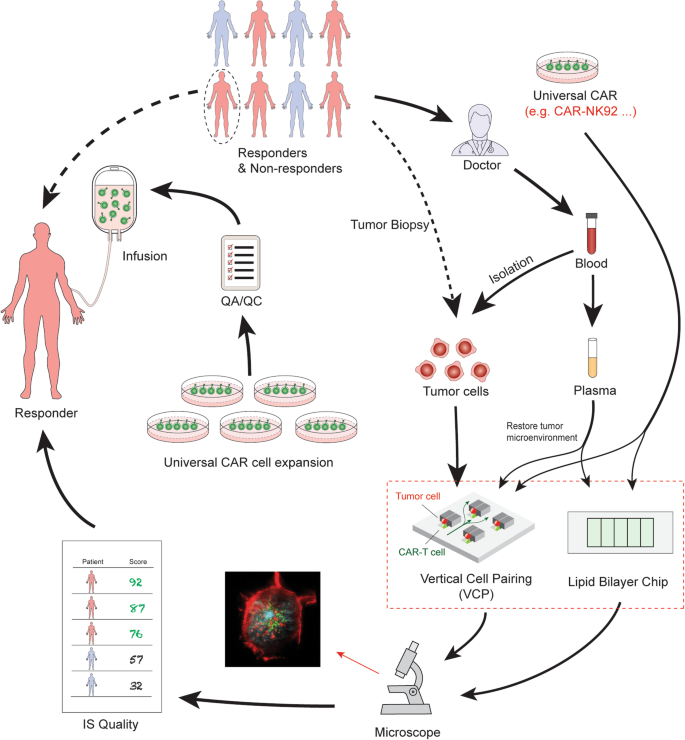
The Role of Immunological Synapse in Predicting the Efficacy of Chimeric Antigen Receptor (CAR) Immunotherapy | Cell Communication and Signaling | Full Text
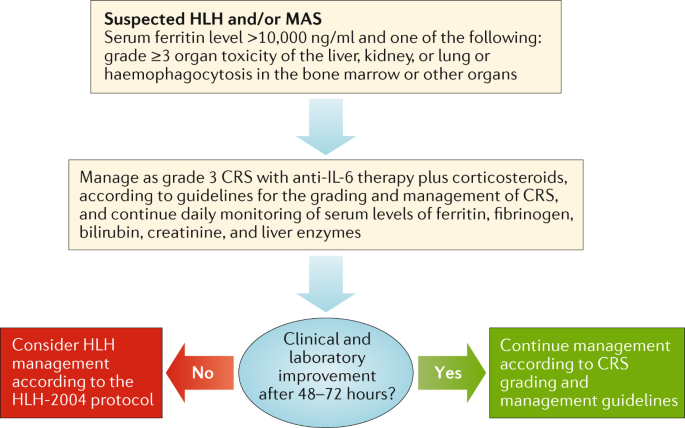
Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy | Nature Reviews Clinical Oncology