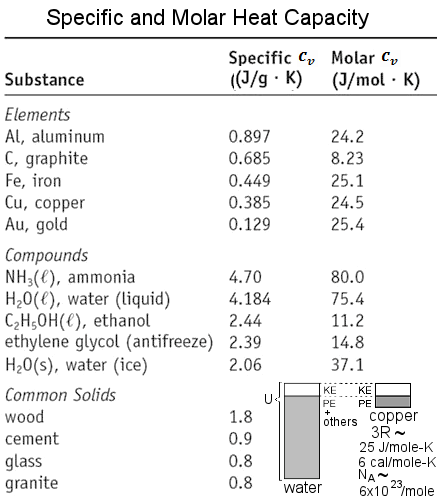
Solved] Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes f... | Course Hero

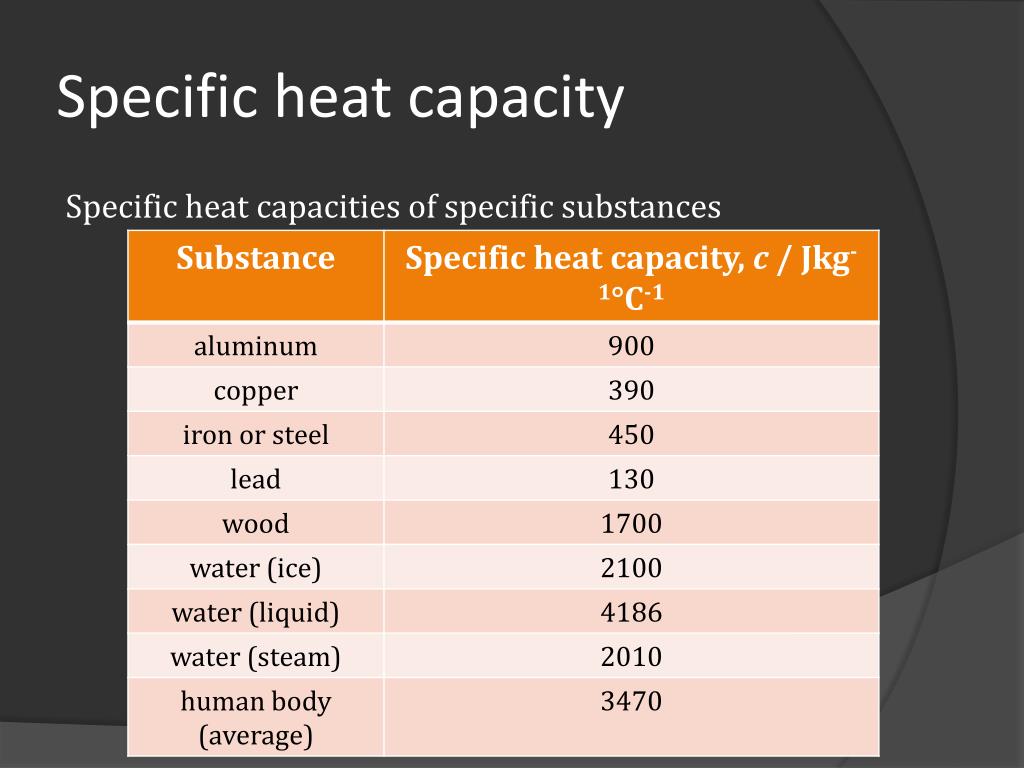
SOLVED:1_ To a 1 kg sample of wood 40kJ of heat is added, and its temperature is found to rise from 20 to 449. What is the specific heat capacity of the

Equilibrium temperatures and specific heat capacities of some wood samples | Download Scientific Diagram

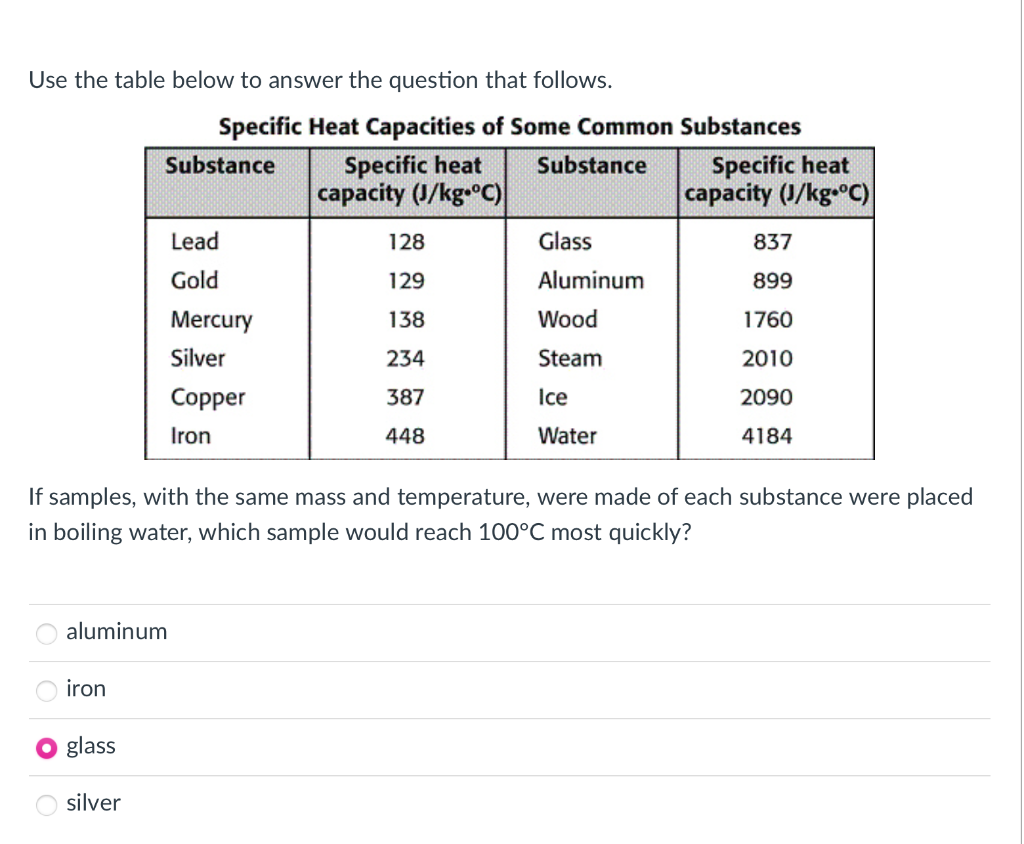

![PDF] Specific Heat Capacity of Wood | Semantic Scholar PDF] Specific Heat Capacity of Wood | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b5a6b9fa21024174fb849f496faf271b114afc67/3-Figure1-1.png)
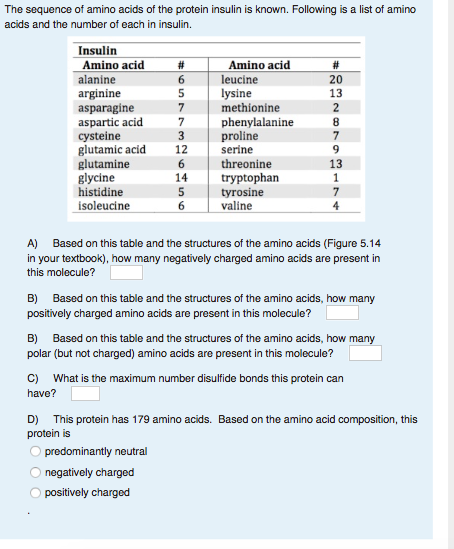


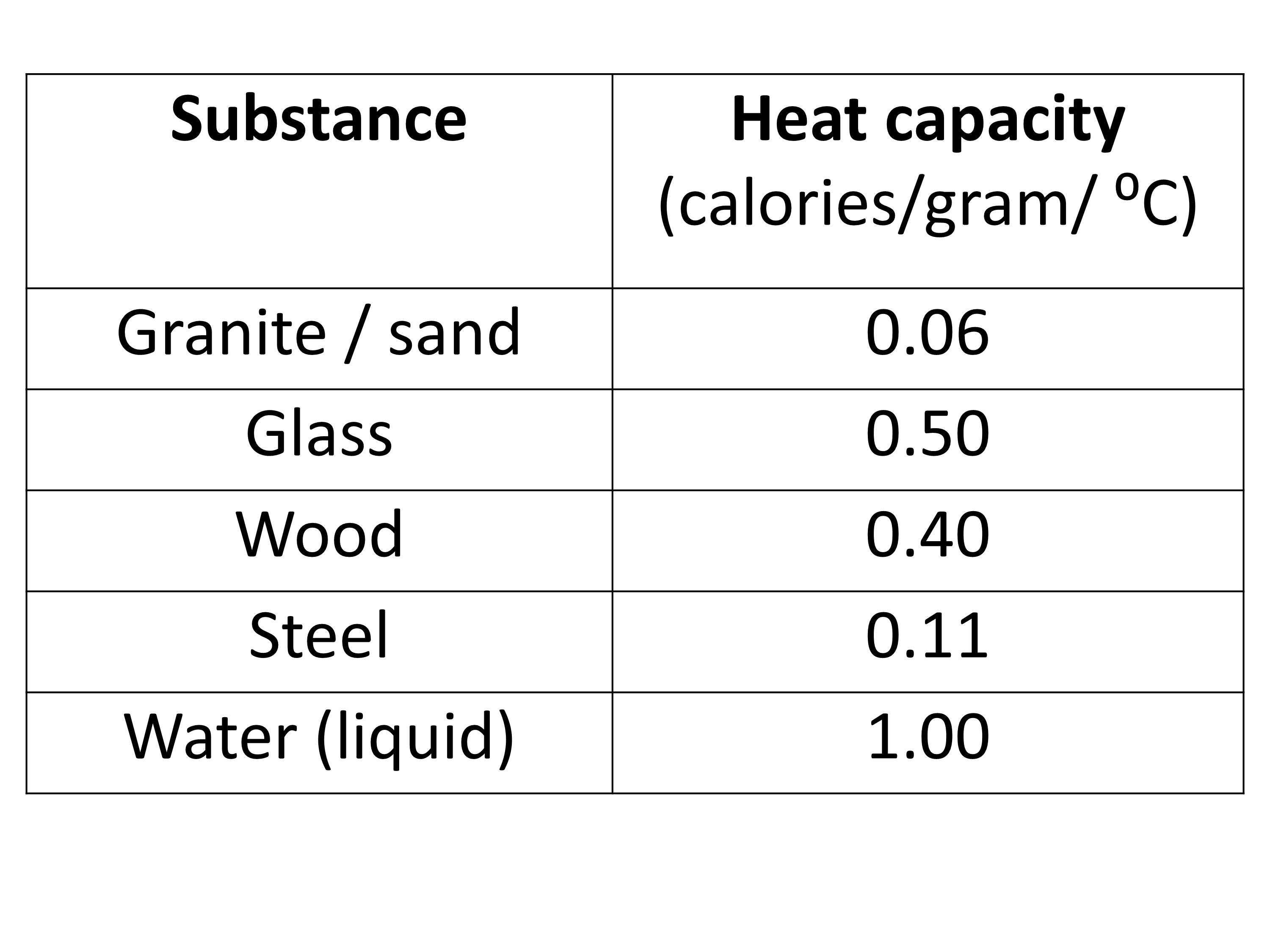

![PDF] Specific Heat Capacity of Wood | Semantic Scholar PDF] Specific Heat Capacity of Wood | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b5a6b9fa21024174fb849f496faf271b114afc67/4-Table2-1.png)